Cut the Noise.
The global customer's first choice for EMI and EMC solutions
Cut the Noise.
The global customer’s first choice for EMI and EMC solutions
Efficiency is not a milestone. It is a continuous process, changing as per newer technologies and environments. At EMIS, we do not stop once a great product is created. We are constantly testing and building efficiency for the ever-evolving world.
Delivering Efficiency across Industries
Delivering Efficiency across Industries
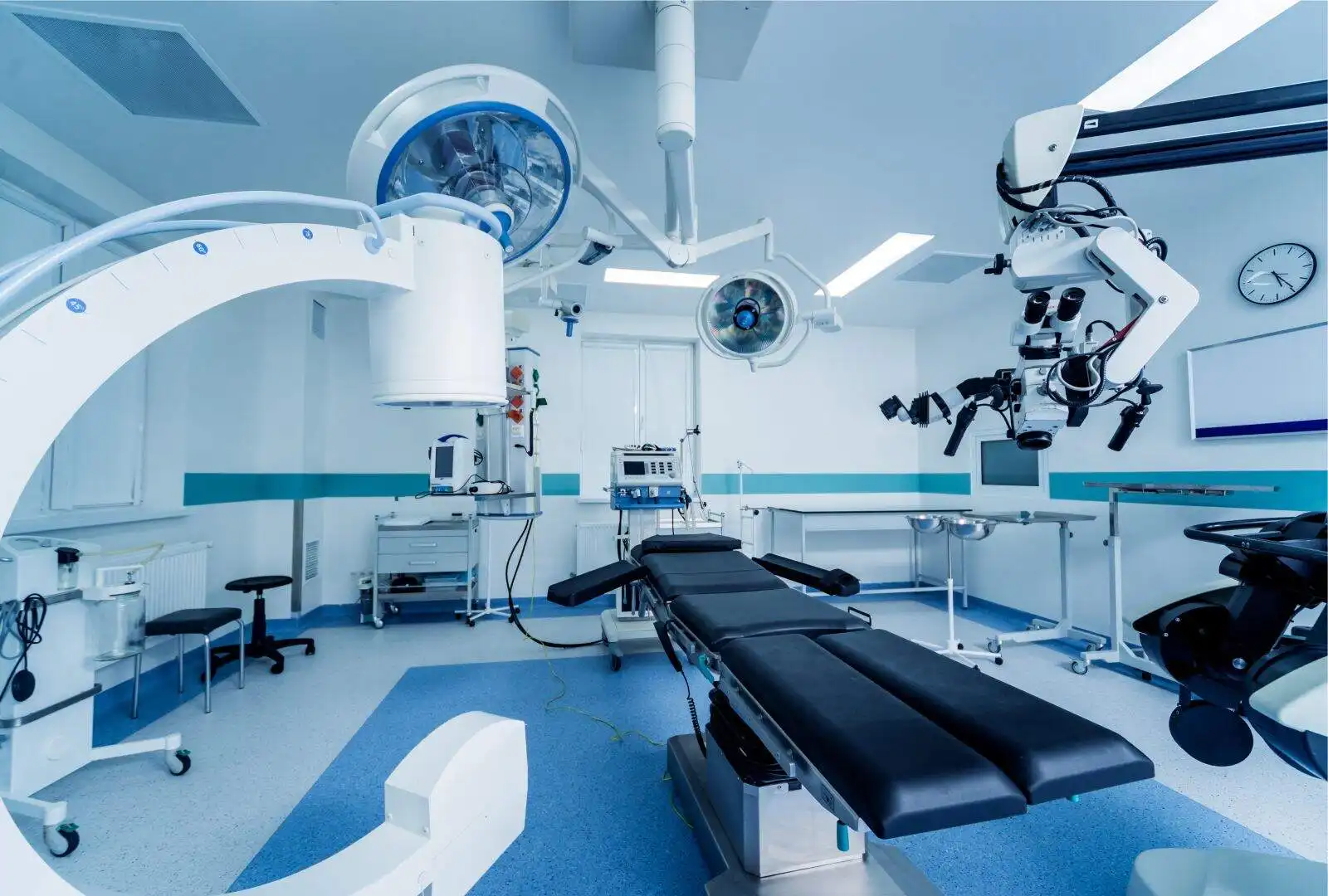
Precision, across the Spectrum
Solving EMI Challenges ranging from 50 Hz to 40 GHz
Services
Scientific solutions that are relevant and adaptable to the evolving requirements and environments


Specifications

 Know more
Know more
Building Exactitude
for World Leaders












News & Insights
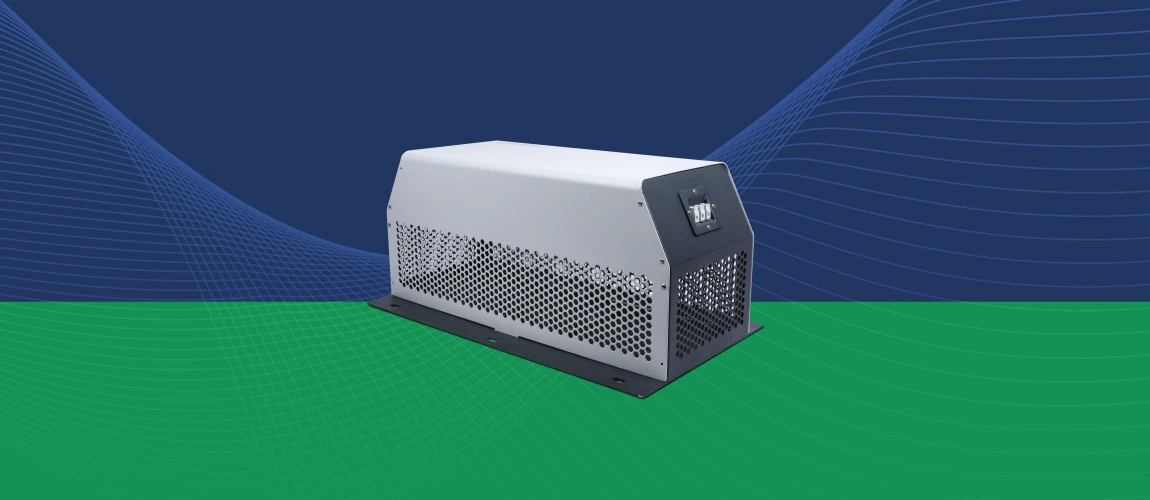
EMI Filters for VFD Applications
What is EMI? Electromagnetic Interference or EMI also commonly called
Brief guide on what are AC Line Reactors & when is it used
The Line Reactors are also called as Line Chokes or Inductors; Lin
Passive Harmonic Filters for Inverters – Three Phase Passive harmonic filters from EMIS
AC motors used in industrial environments are connected to an inverter
DC EMI Filters for Interference and Combat Applications – EMIS
Does DC generate EMI? Why do we need EMI filters for equipment running
Our Global Reach
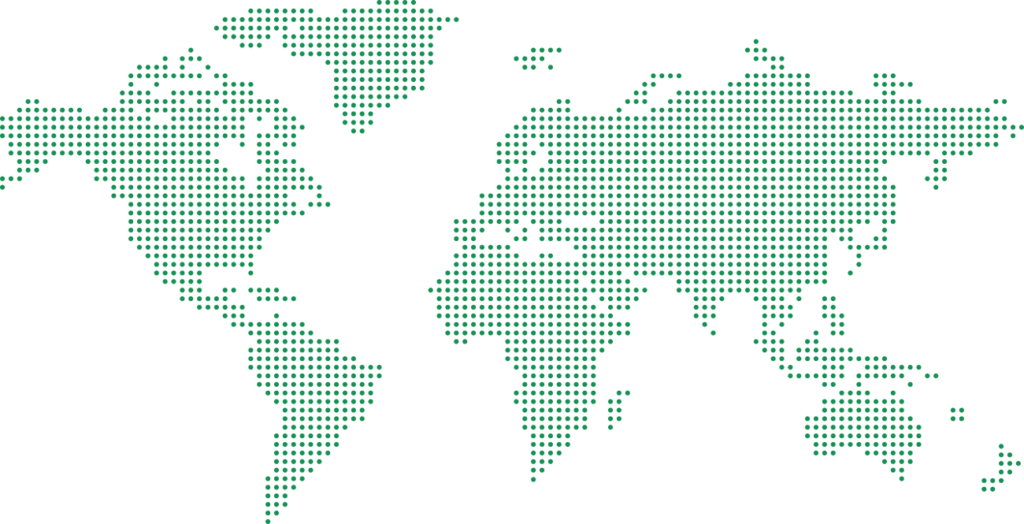
3 Continents | 14 Countries
Asia
India, Israel, Malaysia, Singapore, Thailand, Vietnam
Europe
France, Germany, Italy, Netherlands, Sweden, Spain, Switzerland, UK
America
USA